Cardiotoxicity is one of the main reasons for drug withdrawals, accounting for 45% of all drugs taken off the market between 1994 and 2006. Establishing in vitro screens at the early phases of drug development is critical in preventing late stage failure.
Evotec and wholly owned subsidiary Cyprotex have combined expertise in cardiotoxicity which spans from early discovery through to the clinic so we have a full picture of the entire drug discovery and development continuum and can support you at every stage of your project. The scientific expertise and capabilities can be accessed as fee-for-services or as part of partially or fully integrated programs.
Cardiotoxicity Services
We offer both standard and novel approaches for assessing cardiotoxicity. Both screening and investigative services (non-GLP) and regulatory services (GLP) are available.
Much of our novel research in the field of cardiotoxicity has focused on the area of iPSC-derived cardiomyocytes, whole cell microelectrode array and 3D triculture models.
3D Triculture Models for Studying Cardiotoxicity
We have specific expertise in the development of 3D cell-based models, and much of our research focuses in this area. One important consideration is that the myocardial tissue only comprises 30% cardiomyocytes with the remaining 70% being non-cardiomyocytes (predominantly endothelial and fibroblast cells). These non-cardiomyocytes are essential to myocardial structure and function and emerging evidence suggests that they play a role within drug-induced cardiovascular toxicity.
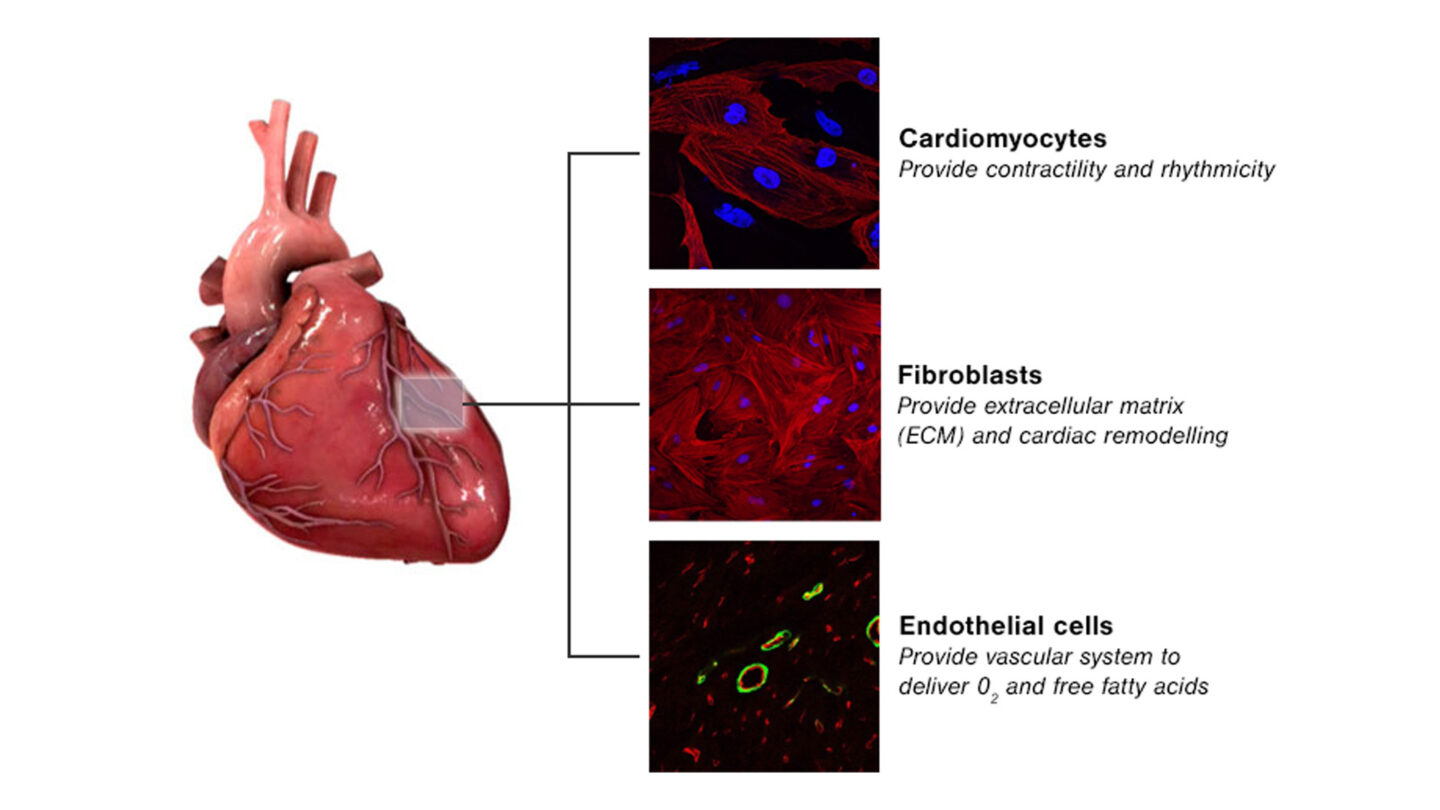
Figure 1
Main cell types present in myocardial tissue.
We have developed cardiac 3D triculture microtissues formed from cardiomyocytes (iPSC-derived), cardiac fibroblasts and cardiac endothelial cells. These models spontaneously beat as shown in the videos below and are amenable to long term culture and so are ideal for repeated dose exposure studies.
The microtissues display uniform size and shape with a diameter of 200µm and whole mount immunofluorescense displays even distribution of key cell markers throughout the microtissues as shown below.
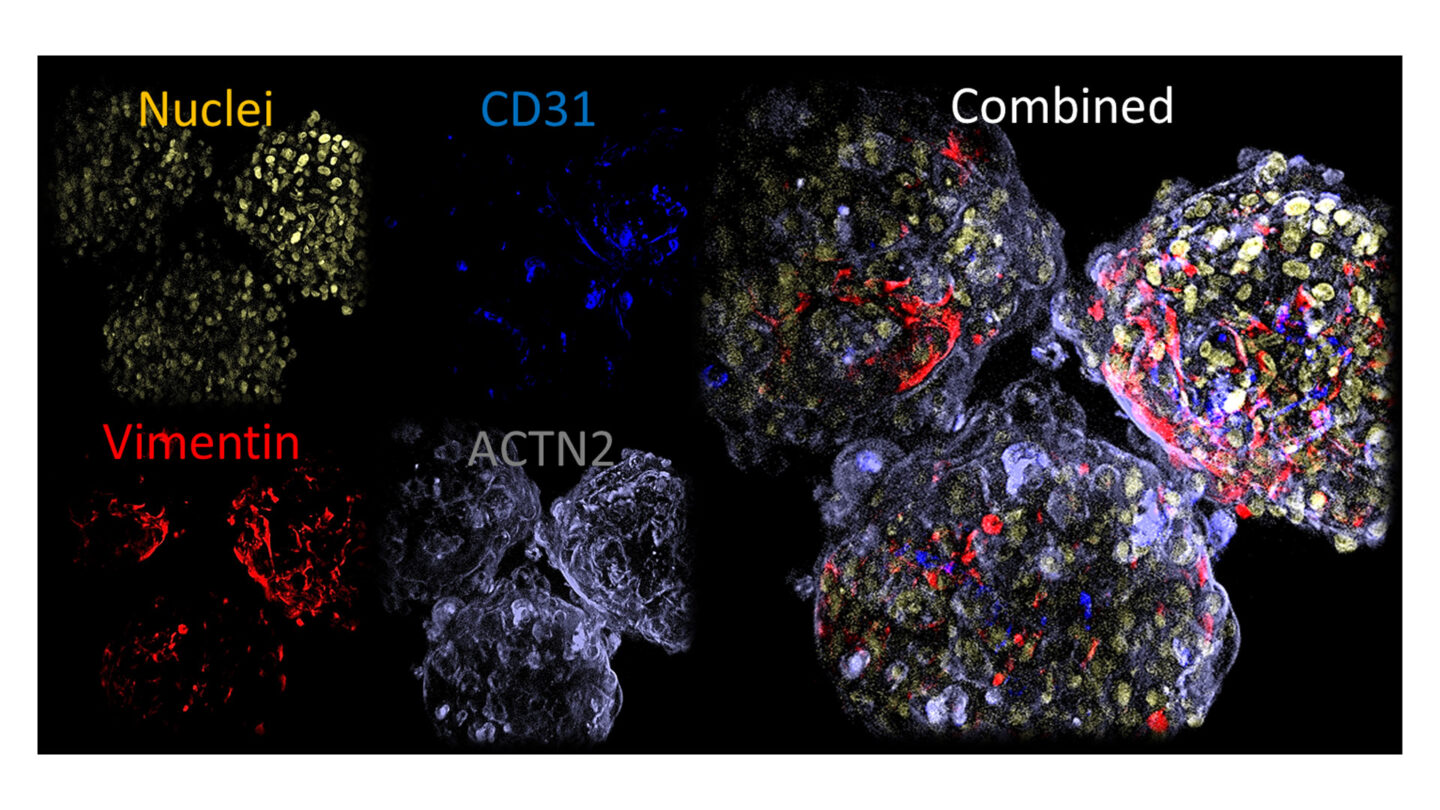
Figure 2
Whole mount immunofluoresence of key markers in microtissues.
We have invested in confocal high content screening for imaging the microtissues and enables a complete analysis of the entire microtissue through Z-stack analysis. These images are illustrated in the videos below.
We have demonstrated how these tissues can be valuable in the following application:
- General structural cardiotoxicity
- Calcium dyshomeostasis
- Hypertrophy
Cardiotox Screen: Cardiac Safety Liability Assessment
We have developed a novel screening assay which combines multi-parametric cellular assays to enable the assessment of both functional and structural cardiotoxicity
The assay comprises of the fast kinetic fluorescent reading of cardiomyocyte calcium transients which have been shown to detect atypical patterns and changes in cell-beating rate caused by hERG, Ca2+ and Na+ channel blockers with high content imaging techniques.
The parameters measured include the following:
- Peak count
- Amplitude
- Frequency
- Full rise time
- Full decay time
- Calcium homeostasis
- Cellular ATP
eCiphr®Cardio - Cardiac Assessment using Microelectrode Array
Cyprotex has considerable expertise in microelectrode array being one of the first companies to launch a high throughput MEA service using iPSC-derived cardiomyocytes (eCiphr®Cardio). Cyprotex has also been heavily involved in the CiPA initiative where as part of the MEA sub-team have a vital role in the evaluation and development of new testing methods.
For eCiphr®Cardio, a highly purified population of cardiomyocytes, which are differentiated from human induced pluripotent stem (iPS) cells, are used. These cells are a mixture of spontaneously electrically-active atrial, nodal and ventricular-like myocytes which beat in synchrony and exhibit expected electrophysiological and biochemical responses upon reference drug exposure.
Using this system, an ECG-like recording can be monitored following test compound exposure.
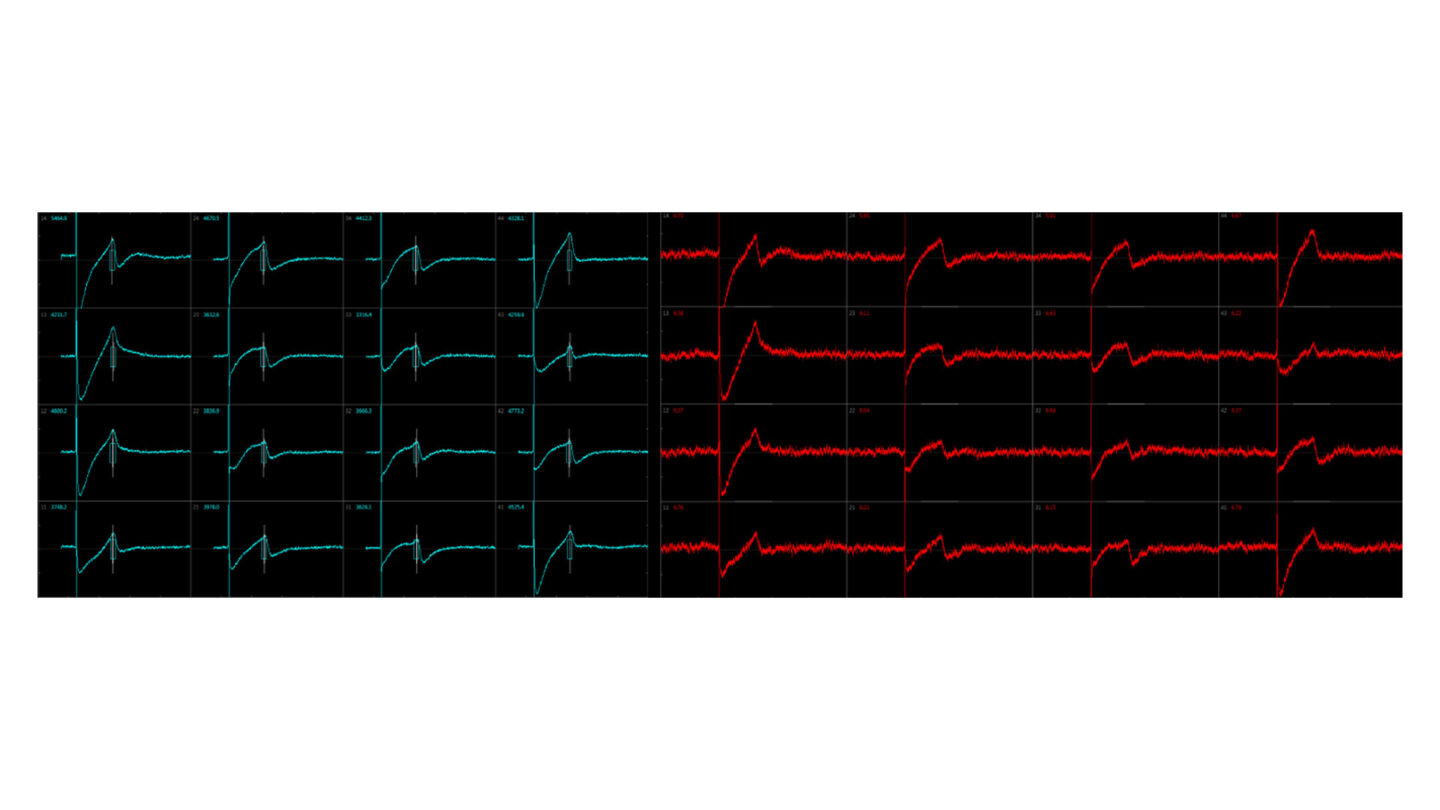
Figure 3
Example traces.
The following parameters can be recorded:
- Beat rate
- Beat period
- Fast Na+ slope
- Fast Na amplitude
- Field potential duration (QT)
Conduction velocity can also be measured. This is defined as the speed at which the signal is translated from a first depolarization event observed in one cell to the depolarization event in an adjacent cell, which results in a wave of activation. In the example below, the effect of lidocaine on the conduction velocity is illustrated. The conduction velocity increases while the amplitude decreases with larger doses of lidocaine.
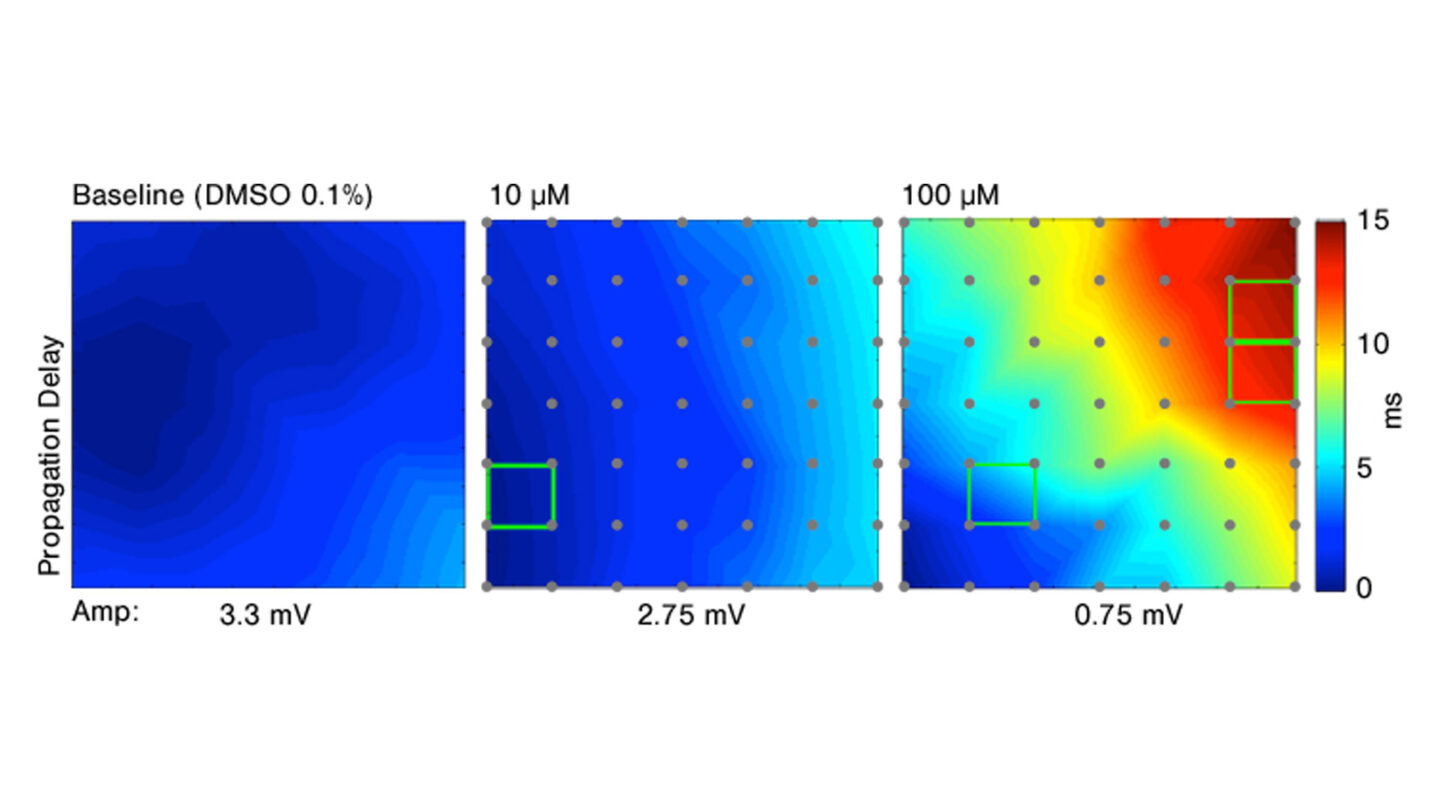
Figure 4
Effect of increasing doses of lidocaine on conduction velocity.
Unlike single ion channel patch clamp measurements, eCiphr®Cardio assesses changes in all major ion channels implicated in an action potential, and so is capable of detecting the effects on the hERG channel, calcium channels, sodium channels and beta adrenergic receptors. By assessing a longer time course, it is also possible to detect hERG trafficking effects using eCiphr®Cardio.
Single Ion Channel Recording
Four essential currents are now recommended within the CiPA panel of ion channels (IKr (rapidly activating delayed rectifier potassium current), INaL (late sodium current), ICaL (L-type calcium current) and INa block (peak sodium current). Evotec is able to offer analysis of these currents using automated patch clamp.
The following ion channels are available (includes CiPA panel and other key ion channels):
- hERG (Ikr)
- Nav1.5 (peak and late current)
- Cav1.2
- KvLQT1/minK (IKs)
- kir2.1 (IK1)
hERG Testing (Screening and Regulatory)
Prolongation of the so-called QT interval, which reflects the time between heart’s ventricular depolarization and repolarization is a common reason for cardiotoxicity. Virtually all drugs that prolong QT interval block the delayed rectifier K+ current (Ikr), which is responsible for repolarization. The prolongation of the QT interval has been associated with torsade de pointes (TdP), which may lead to ventricular arrhythmia and consequently sudden cardiac death.
However, TdP is not always associated with QT interval prolongation and not all Ikr blockers cause QT prolongation or TdP. It is thus critical to design assays that can examine the effects of drug candidates on the entire action potential and thereby uncover all potential mechanisms behind drug-induced cardiotoxicity.
Utilizing cell-based assays, Evotec and wholly owned subsidiary Cyprotex are able to assess single ion channel effects by measuring hERG inhibition in an automated patch clamp system (QPatch HTX System or the SyncroPatch 384PE), as well as whole cell electrophysiology using microelectrode array (eCiphr®Cardio).
Both screening and regulatory hERG assessments are available.

