Introduction
Determine potential hERG inhibition of new chemical entities using the QPatch HTX or the SyncroPatch 384PE.
We deliver consistent, high quality data with the flexibility to adapt protocols based on specific customer requirements.
hERG Inhibition for Assessing Potential Cardiotoxicity
- The human ether-a-go-go related gene (hERG) encodes the inward rectifying voltage gated potassium channel in the heart (IKr) which is involved in cardiac repolarization.
- Inhibition of the hERG current causes QT interval prolongation resulting in potentially fatal ventricular tachyarrhythmia called Torsade de Pointes.
- A number of drugs have been withdrawn from late stage clinical trials due to these cardiotoxic effects, therefore it is important to identify inhibitors early in drug discovery.
- The hERG Safety service is performed at our parent company, Evotec, and is a cell-based assay which employs the QPatch HTX System (Sophion Bioscience A/S) or the SyncroPatch 384PE (Nanion Technologies) as automated patch clamp electrophysiology measurements.
- The QPatch HTX and the SyncroPatch 384PE systems deliver high quality, accurate and sensitive data which are comparable with the traditional single cell patch clamp method.
Protocol
hERG Safety Protocol
Data
Recording System for the hERG QPatch HTX Assay
The QPatch HTX is a high-throughput and automated electrophysiology platform. The QPatch performs up to 48 independent patch clamp experiments in parallel in a 48-channel chip array with microfluidic chambers (QPlate). QPatch HTX system uses the general principle of planar patch clamp: each chamber of the QPlate consists of two compartments containing extra- and intra-solutions, separated by a silicon-based glass planar substrate that has a micron-sized aperture.
Cells in suspension are placed in the wells and interact with the glass to form stable giga-seal at the aperture. After giga-seal formation, whole-cell configuration is achieved by physical rupture of the cell-attached patch, then pre-programmed voltage-clamp steps are applied and recording begins.
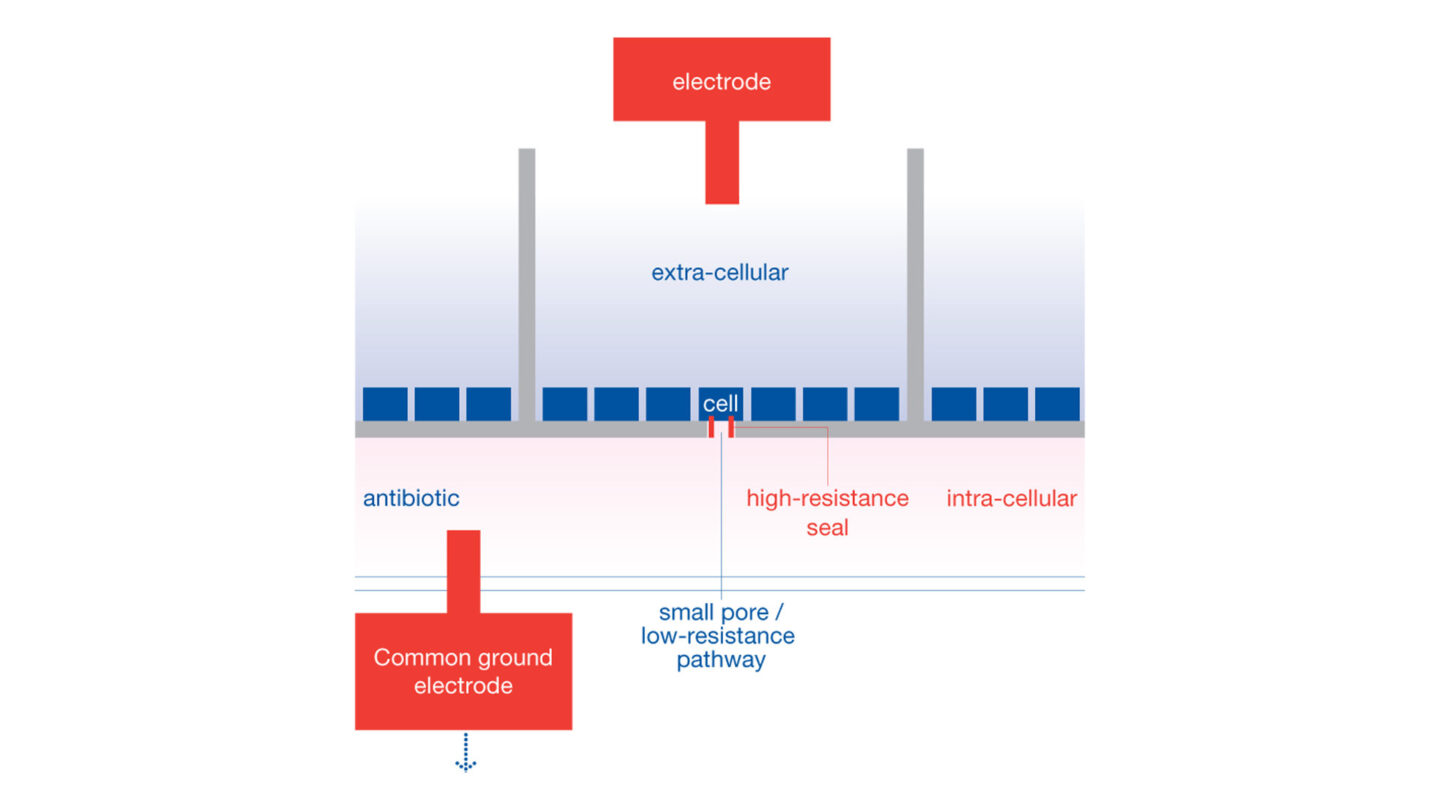
Figure 1
QPlate chamber configuration for QPatch system. Reproduced with permission from Sophion.
Voltage protocol for the hERG QPatch HTX assay
The voltage protocol is run and recorded continuously during the experiment. The stability of recording is assessed through initial wash with extracellular solution alone. The vehicle, corresponding to test substances perfusion solutions, is applied for 3 min followed by the test substance. The test substances are applied in triplicate, to ensure adequate mixing, sequentially at increasing concentrations to at least two cells. The standard combined exposure time is 5 min.
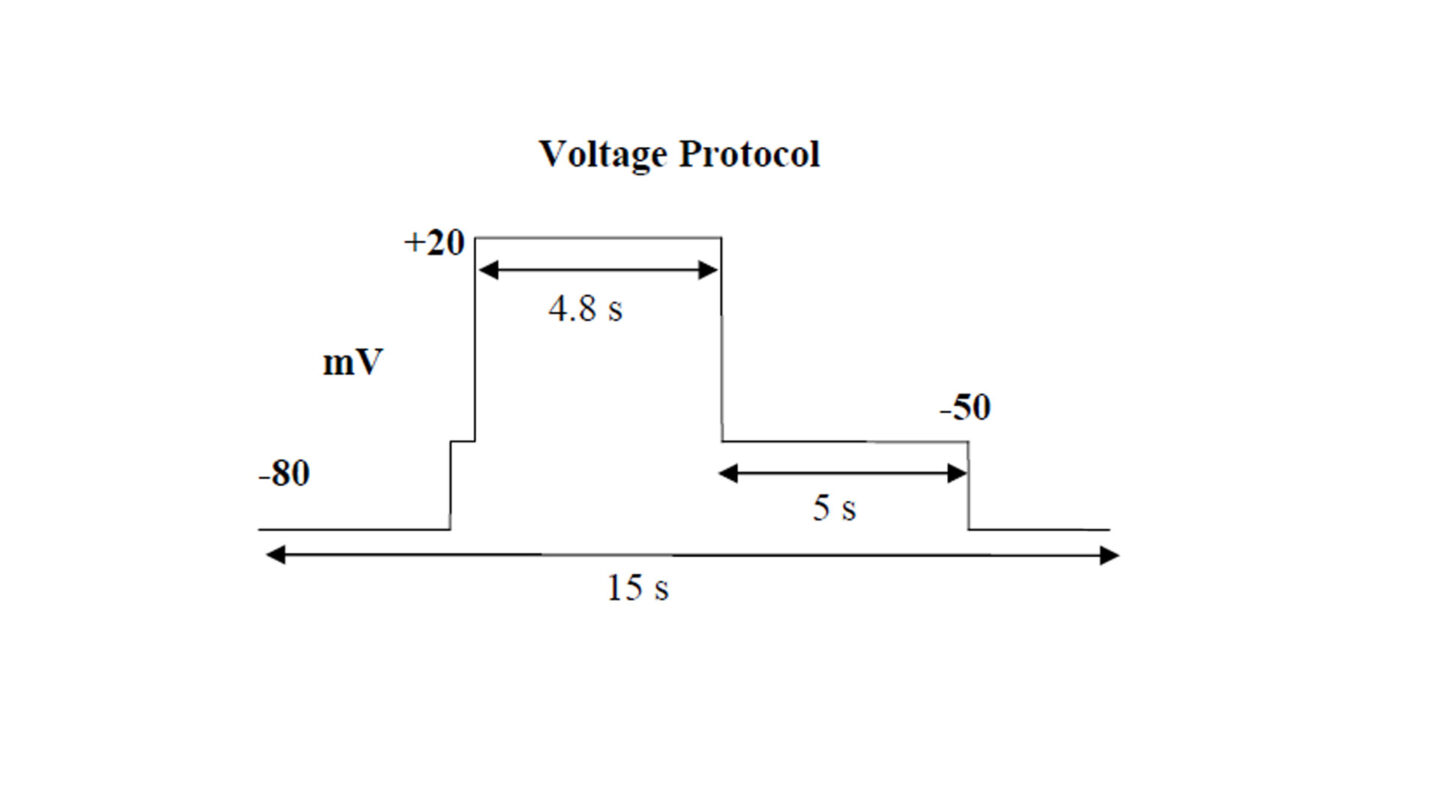
Figure 2
Schematic diagram of the voltage protocol used in the QPatch experiment.
Study Design for the QPatch HTX Assay
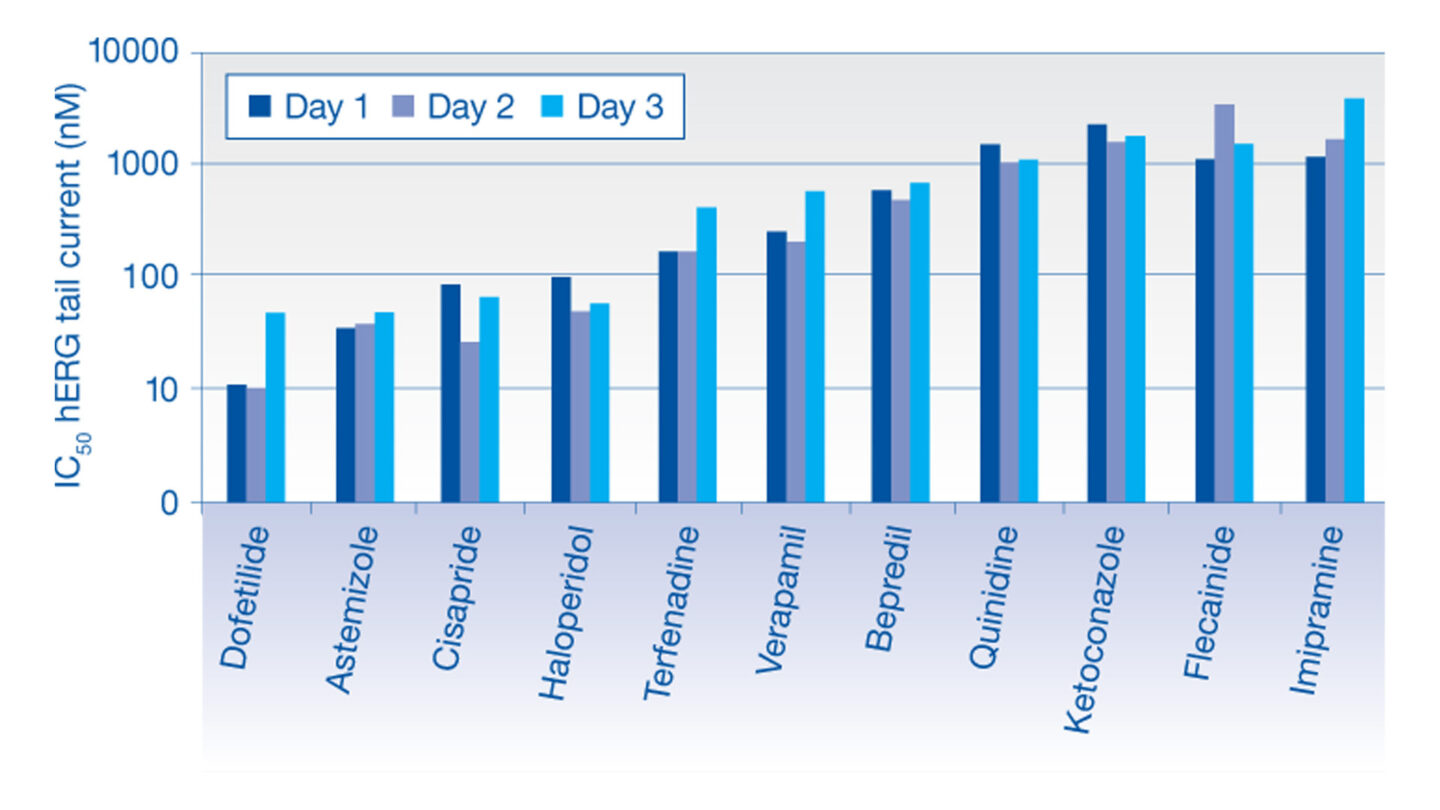
Figure 3
Diagram of study design illustrating the treatment group for the three test concentrations.
Example of Current Recording for the QPatch HTX
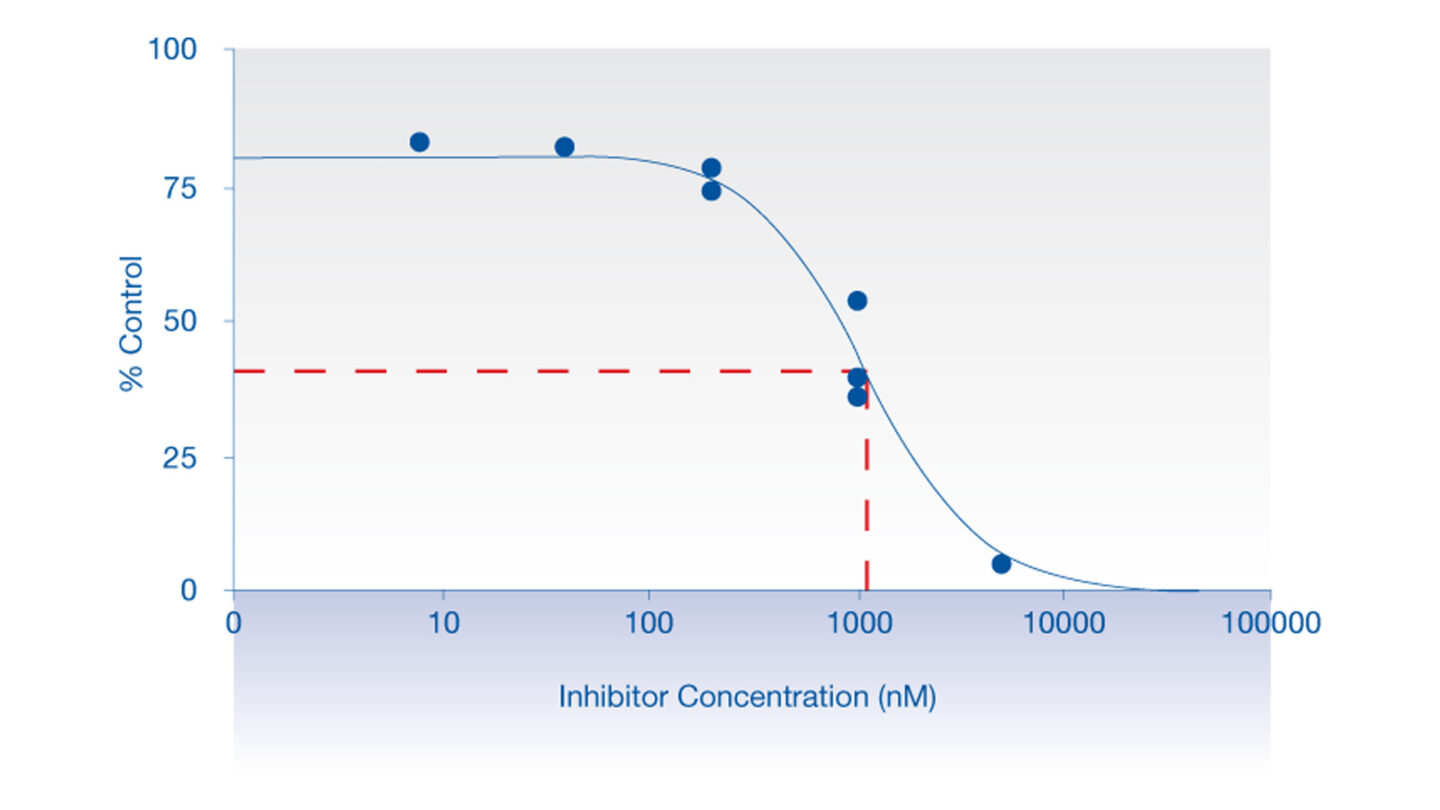
Figure 4
Example of current recording on the QPatch HTX.
Recording System for the hERG SyncroPatch 384PE Assay
The SyncroPatch 384 Patch Engine (PE) automated patch clamp instrument simultaneously performs electrophysiology measurements for multiple single cells in specialised 384 well plates. After initiating the experiment, cell catching, sealing, perforated-cell formation, liquid application, recording, and data acquisition are performed sequentially. Initially, the chip is primed with appropriate extracellular and intracellular solutions. The suspended single cells, stored in a cell hotel reservoir with orbital shaking speed at 200 rpm, are aspirated from the reservoir, pipetted into the planar 384-well patch-clamp chip, and entrapped in the holes of the wells by an automatically applied vacuum. Seal generation, the establishment of the perforated cell mode and also the electrophysiological recordings are controlled by PatchControl 384. Once a stable patch has been achieved, recording commences in voltage-clamp mode.
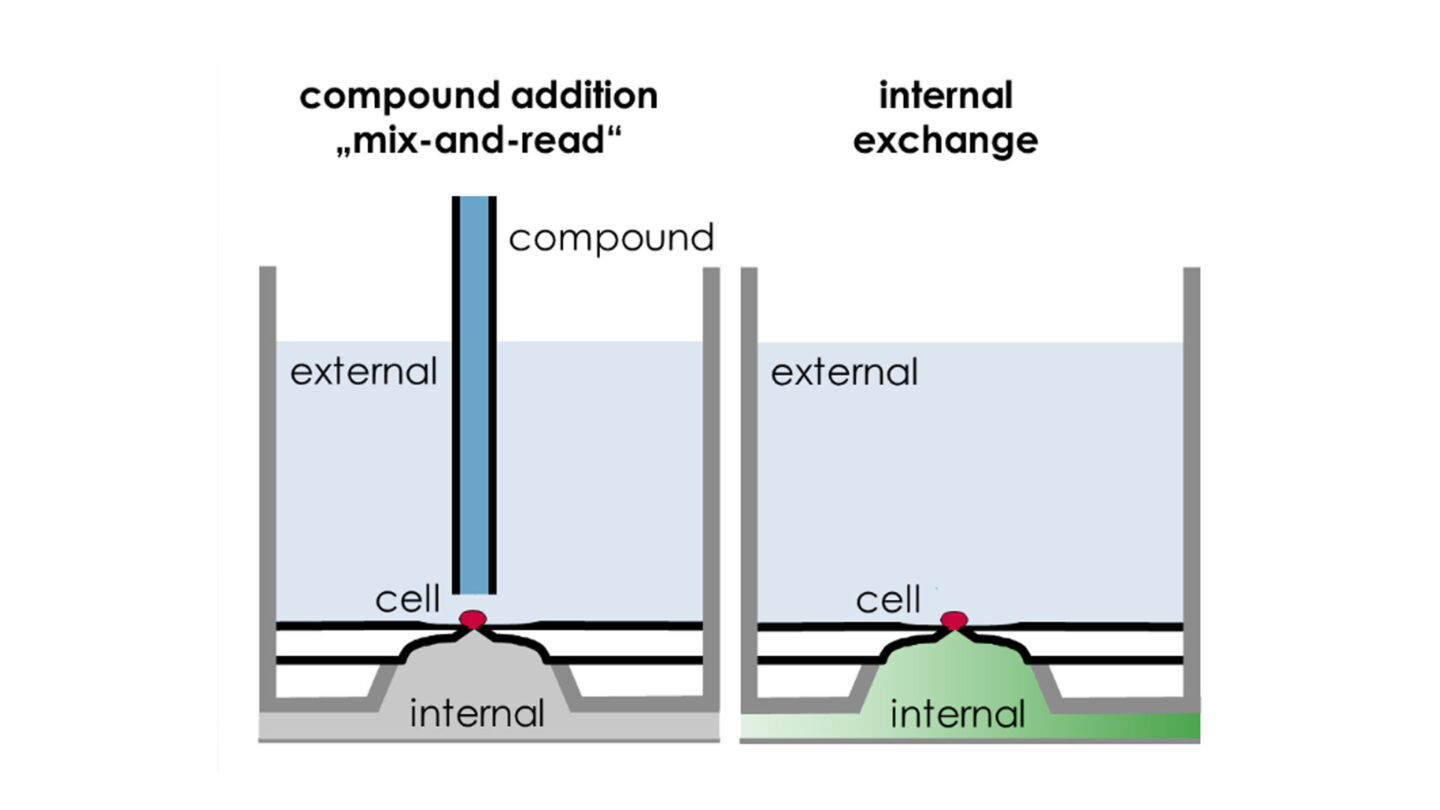
Figure 5
SyncroPatch 384PE: Examples of pharmacological application configurations. Reproduced with permission from Nanion.
Voltage Protocol for the hERG SyncroPatch 384PE Assay
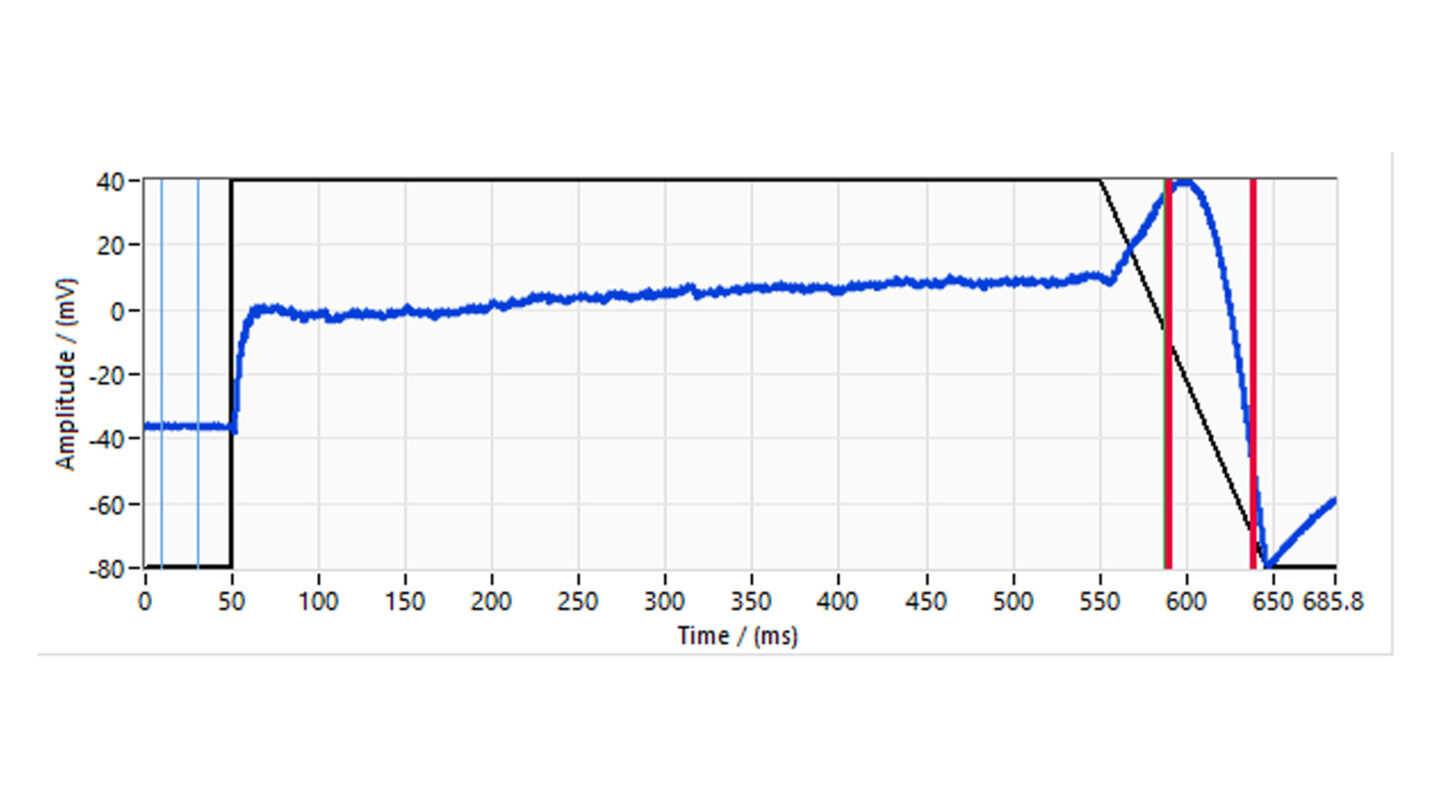
Figure 6
Diagram of study design illustrating the treatment group for the three test concentrations.
Study Design for the hERG SyncroPatch 384PE Assay
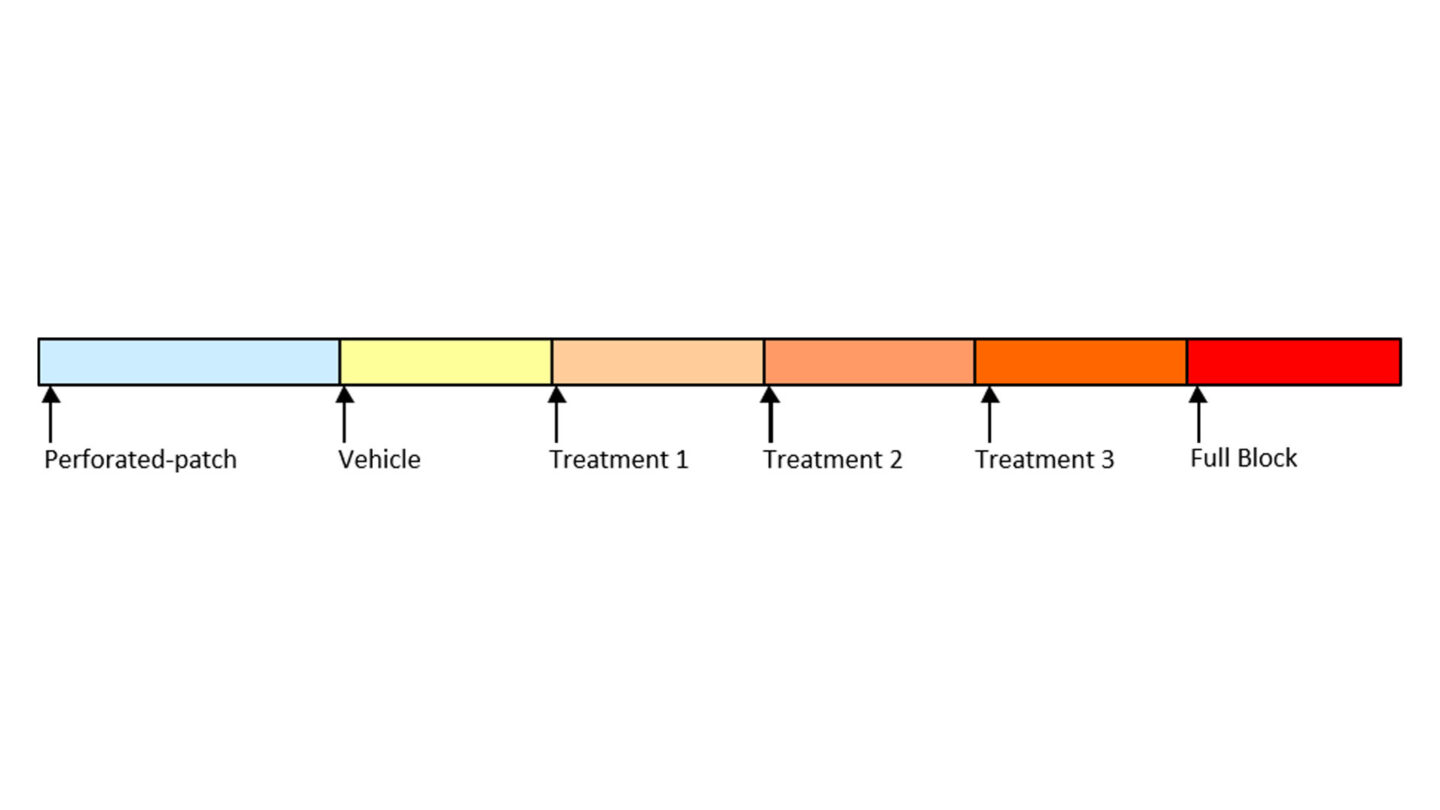
Figure 7
Diagram of study design for the SynchroPatch 384PE assay illustrating the treatment group for the three test concentrations.
Example of Current Recording for the SyncroPatch 384PE
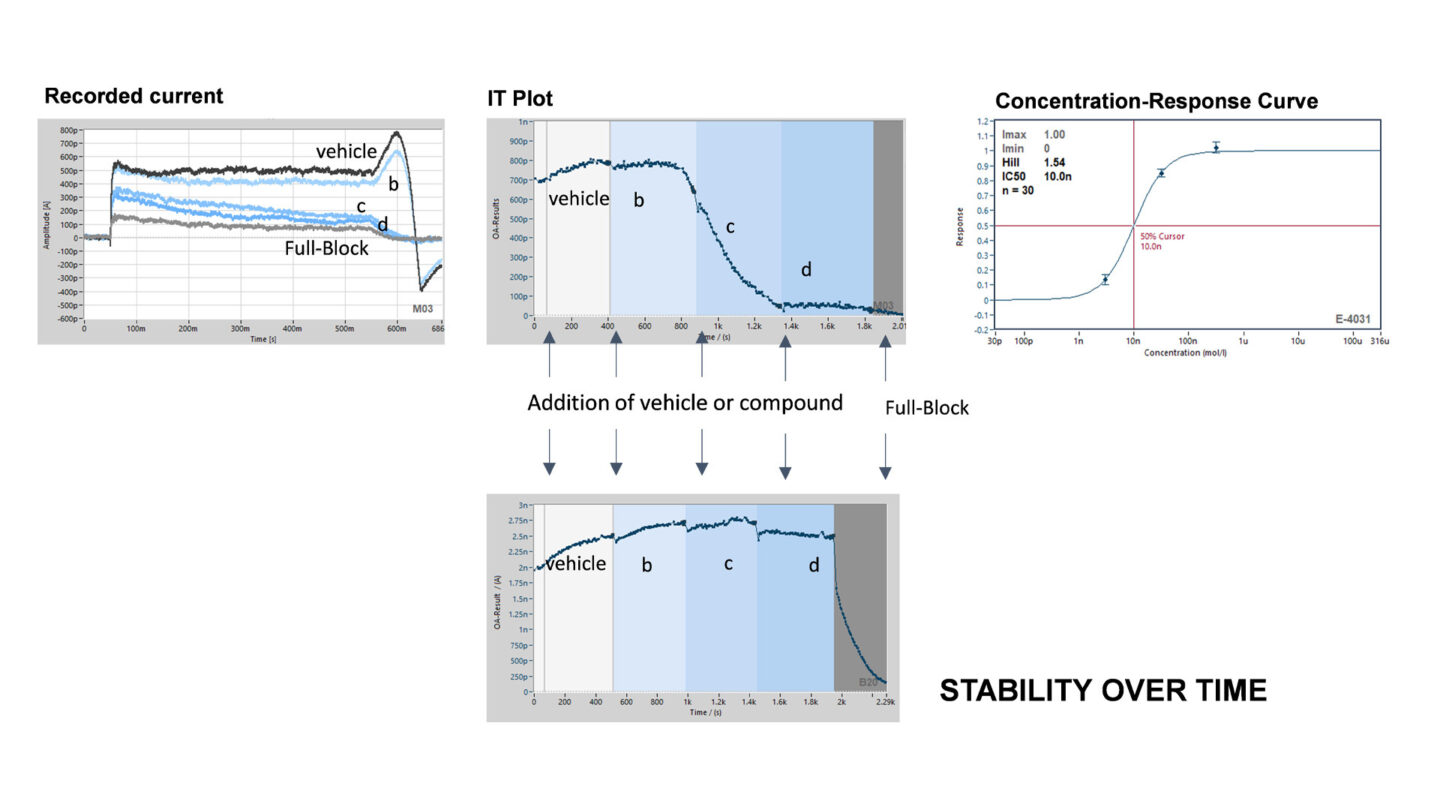
Figure 8
Example of current recording on the SyncroPatch 384PE.
Data from the hERG Safety Assay
Q&A
Why is it important to investigate hERG inhibition?
The human ether-a-go-go related gene (hERG) encodes the inward rectifying voltage gated potassium channel in the heart (IKr) which is involved in cardiac repolarization. Inhibition of the hERG current causes QT interval prolongation resulting in potentially fatal ventricular tachyarrhythmia called Torsade de Pointes. A number of drugs have been withdrawn from late stage clinical trials due to these cardiotoxic effects, therefore it is important to identify inhibitors early in drug discovery1.
Although the focus on hERG block and QT prolongation has largely eliminated new drugs entering the market with unanticipated potential for torsade, it has important limitations and may have led to stopping the development of potentially valuable therapeutics. Therefore, a Comprehensive In vitro Proarrhythmia Assay (CiPA) was proposed as a new paradigm. An international multi-disciplinary team of regulatory, industry and academic scientists are working together to develop and validate a set of predominantly nonclinical assays and methods that eliminate the need for the thorough-QT study and enable a more precise prediction of clinical proarrhythmia risk.
Please provide an overview of the hERG Safety assay.
The effect of test compounds on the hERG cardiac ion channel, expressed in mammalian cells (HEK-293), is assessed using the electrophysiology platforms QPatch HTX and/or SyncroPatch 384PE. These are automated electrophysiology systems that follows the general principles of conventional patch-clamping. Test compounds are tested at a single concentration or at 3 concentrations (cumulative concentration-response). The percent change in hERG tail-current is calculated and used, in case of concentration-response tests, to calculate an IC50 value (test compound concentration that produces 50 % inhibition). We deliver percentage inhibition and, where appropriate, an IC50 for the test compound.
What controls are included in the hERG assay?
Only cells with a seal resistance greater than 100 MOhm (for QPatch system) or 50 MOhm (for SyncroPatch system) and a pre-compound current of at least 0.2 nA are used to evaluate hERG blockade. E-4031, a known hERG inhibitor, is used as a positive control for the experiment. DMSO is included as a negative control.
How does in vitro hERG inhibition relate to in vivo cardiotoxicity?
The hERG channel inhibition assay is a highly sensitive measurement which will identify compounds exhibiting cardiotoxicity related to hERG inhibition in vivo. It is important to note, however that not all compounds which inhibit hERG activity in vitro will proceed to cause cardiotoxicity in vivo. The relevance of the in vitro data will be dependent on other factors such as the plasma concentrations reached in vivo.
On a cellular level, cardiac repolarization is a complex process representing a fine balance of multiple time- and voltage-dependent inward and outward currents. At an organ level, differences in current densities within the ventricles are responsible for the heterogeneity of ventricular repolarization that gives rise to the QT interval. Recent data suggest that drugs affect more cardiac currents than previously expected: automated patch-clamp systems have demonstrated the promiscuity of multiple non-cardiovascular drugs, including blockade of cardiac IKr, ICaL (the L-type Ca2+ current) and INa (a Na+ current).
References
1) Haverkamp W et al. (2000) The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drugs: clinical and regulatory implications. Eur Heart J 21(15); 1216-31
2) Okada J et al. (2015) Screening system for drug-induced arrhythmogenic risk combining a patch clamp and heart simulator. Sci Adv 1(4); e1400142
3) Polonchuk L (2014) Industrializing electrophysiology: HT automated patch clamp on SyncroPatch 96 using instant frozen cells. Methods in Molec Biol 1183; 81-92
4) Obergrussberger A et al (2016) Automated patch clamp meets high-throughput screening: 384 cells recorded in parallel on a planar patch clamp module. J Lab Autom 21(6); 779-793
5) Crumb WJ et al (2016) An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J Pharmacol Toxicol Methods 81; 251-262

